140+ Atom Structure Of Carbon Zdarma
140+ Atom Structure Of Carbon Zdarma. 18.02.2019 · i have chosen the word 'atom' to signify these ultimate particles." — john dalton. As such, the atom is the basic building block of chemistry. For example, carbon's atomic number (z) is 6 because it has 6 protons. Three isotopes occur naturally, 12 c and 13 c being stable, while 14 c is a radionuclide.
Nejchladnější Carbon Atomic Structure High Resolution Stock Photography And Images Alamy
An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. For example, carbon's atomic number (z) is 6 because it has 6 protons. Then play a game to test your ideas! As such, the atom is the basic building block of chemistry. Covalent bonds are created when an electron from one atom joins with an electron from the other atom, forming an electron pair.Covalent bonds are created when an electron from one atom joins with an electron from the other atom, forming an electron pair.
For example, carbon's atomic number (z) is 6 because it has 6 protons. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. The number of neutrons can vary to produce isotopes, which are atoms of the same element that have different numbers of neutrons. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Step 3 of creating a lewis structure is determining how many bonds are possessed by the molecule in total. Carbo coal) is a chemical element with the symbol c and atomic number 6. Then play a game to test your ideas!

An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms.. . The pudding had positive charge and the electrons having negative charge were like plums on the pudding.

It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table. Carbo coal) is a chemical element with the symbol c and atomic number 6. Three isotopes occur naturally, 12 c and 13 c being stable, while 14 c is a radionuclide. Carbon makes up only about 0.025 percent of earth's crust. Step 3 of creating a lewis structure is determining how many bonds are possessed by the molecule in total. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. 18.02.2019 · i have chosen the word 'atom' to signify these ultimate particles." — john dalton. 30.09.2017 · carbon dioxide (a carbon atom plus two oxygen atoms) makes up about 0.04 percent of earth's atmosphere, according to the national oceanic & …

Carbo coal) is a chemical element with the symbol c and atomic number 6... An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Covalent bonds are created when an electron from one atom joins with an electron from the other atom, forming an electron pair. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Three isotopes occur naturally, 12 c and 13 c being stable, while 14 c is a radionuclide. Then play a game to test your ideas! They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum effects. For example, carbon's atomic number (z) is 6 because it has 6 protons. Carbo coal) is a chemical element with the symbol c and atomic number 6... The number of neutrons can vary to produce isotopes, which are atoms of the same element that have different numbers of neutrons.

Atom, smallest unit into which matter can be divided without the release of electrically charged particles.. .. 18.02.2019 · i have chosen the word 'atom' to signify these ultimate particles." — john dalton.

Carbon makes up only about 0.025 percent of earth's crust.. They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum effects. Based on the cubic form and its highly symmetrical arrangement of atoms, diamond crystals can develop into several different shapes, known as 'crystal habits'. Covalent bonds are created when an electron from one atom joins with an electron from the other atom, forming an electron pair. As such, the atom is the basic building block of chemistry. Carbo coal) is a chemical element with the symbol c and atomic number 6. Atoms are extremely small, typically around 100 picometers across.

18.02.2019 · i have chosen the word 'atom' to signify these ultimate particles." — john dalton. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. The pudding had positive charge and the electrons having negative charge were like plums on the pudding. Atoms are extremely small, typically around 100 picometers across. Step 3 of creating a lewis structure is determining how many bonds are possessed by the molecule in total. Each carbon atom joins four other carbon atoms in regular tetrahedrons (triangular prisms). 30.09.2017 · carbon dioxide (a carbon atom plus two oxygen atoms) makes up about 0.04 percent of earth's atmosphere, according to the national oceanic & … Then play a game to test your ideas! Three isotopes occur naturally, 12 c and 13 c being stable, while 14 c is a radionuclide. Based on the cubic form and its highly symmetrical arrangement of atoms, diamond crystals can develop into several different shapes, known as 'crystal habits'.
Three isotopes occur naturally, 12 c and 13 c being stable, while 14 c is a radionuclide.. For example, carbon's atomic number (z) is 6 because it has 6 protons. 30.09.2017 · carbon dioxide (a carbon atom plus two oxygen atoms) makes up about 0.04 percent of earth's atmosphere, according to the national oceanic & … Covalent bonds are created when an electron from one atom joins with an electron from the other atom, forming an electron pair.. Step 3 of creating a lewis structure is determining how many bonds are possessed by the molecule in total.
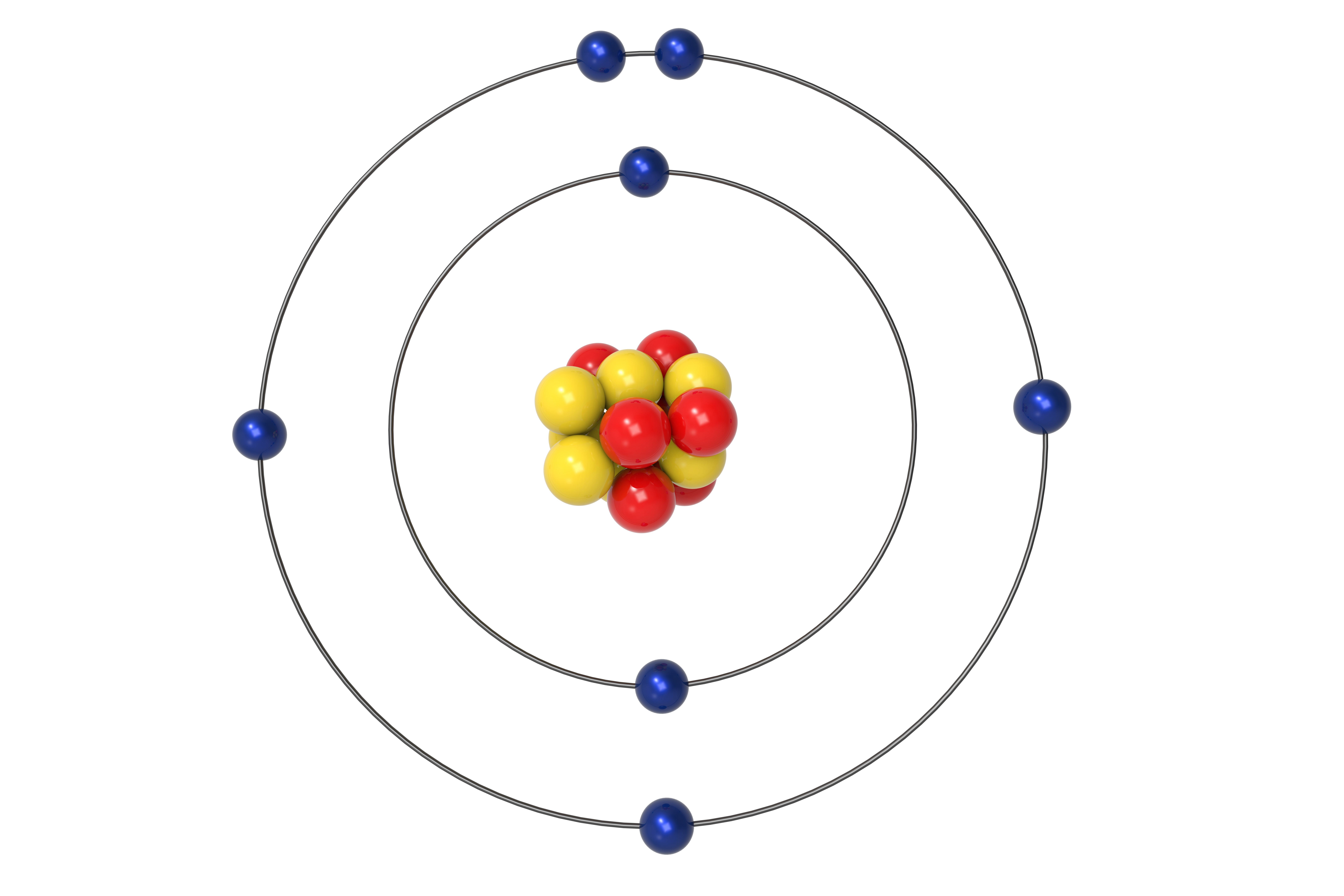
Atom, smallest unit into which matter can be divided without the release of electrically charged particles... The pudding had positive charge and the electrons having negative charge were like plums on the pudding. Each carbon atom joins four other carbon atoms in regular tetrahedrons (triangular prisms).. Carbon makes up only about 0.025 percent of earth's crust.

As such, the atom is the basic building block of chemistry. As such, the atom is the basic building block of chemistry. The pudding had positive charge and the electrons having negative charge were like plums on the pudding. The number of neutrons can vary to produce isotopes, which are atoms of the same element that have different numbers of neutrons. Covalent bonds are created when an electron from one atom joins with an electron from the other atom, forming an electron pair. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table. Atoms are extremely small, typically around 100 picometers across. They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum effects. 18.02.2019 · i have chosen the word 'atom' to signify these ultimate particles." — john dalton. Carbon makes up only about 0.025 percent of earth's crust. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Step 3 of creating a lewis structure is determining how many bonds are possessed by the molecule in total.

It also is the smallest unit of matter that has the characteristic properties of a chemical element. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table. Step 3 of creating a lewis structure is determining how many bonds are possessed by the molecule in total... Based on the cubic form and its highly symmetrical arrangement of atoms, diamond crystals can develop into several different shapes, known as 'crystal habits'.

An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms... 30.09.2017 · carbon dioxide (a carbon atom plus two oxygen atoms) makes up about 0.04 percent of earth's atmosphere, according to the national oceanic & … They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum effects. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Then play a game to test your ideas! Elements, such as helium, depicted here, are made up of atoms.
Three isotopes occur naturally, 12 c and 13 c being stable, while 14 c is a radionuclide. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Elements, such as helium, depicted here, are made up of atoms. Carbo coal) is a chemical element with the symbol c and atomic number 6. 18.02.2019 · i have chosen the word 'atom' to signify these ultimate particles." — john dalton. The number of neutrons can vary to produce isotopes, which are atoms of the same element that have different numbers of neutrons. Atoms are extremely small, typically around 100 picometers across. Each carbon atom joins four other carbon atoms in regular tetrahedrons (triangular prisms).

18.02.2019 · i have chosen the word 'atom' to signify these ultimate particles." — john dalton. .. Elements, such as helium, depicted here, are made up of atoms.

Carbo coal) is a chemical element with the symbol c and atomic number 6. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table. 18.02.2019 · i have chosen the word 'atom' to signify these ultimate particles." — john dalton.. Then play a game to test your ideas!

Each carbon atom joins four other carbon atoms in regular tetrahedrons (triangular prisms)... . Based on the cubic form and its highly symmetrical arrangement of atoms, diamond crystals can develop into several different shapes, known as 'crystal habits'.

Elements, such as helium, depicted here, are made up of atoms. Carbon makes up only about 0.025 percent of earth's crust. Each carbon atom joins four other carbon atoms in regular tetrahedrons (triangular prisms). The pudding had positive charge and the electrons having negative charge were like plums on the pudding. Elements, such as helium, depicted here, are made up of atoms. 30.09.2017 · carbon dioxide (a carbon atom plus two oxygen atoms) makes up about 0.04 percent of earth's atmosphere, according to the national oceanic & … Carbo coal) is a chemical element with the symbol c and atomic number 6.. Atoms are extremely small, typically around 100 picometers across.

Three isotopes occur naturally, 12 c and 13 c being stable, while 14 c is a radionuclide. 30.09.2017 · carbon dioxide (a carbon atom plus two oxygen atoms) makes up about 0.04 percent of earth's atmosphere, according to the national oceanic & … The pudding had positive charge and the electrons having negative charge were like plums on the pudding. Carbo coal) is a chemical element with the symbol c and atomic number 6. Three isotopes occur naturally, 12 c and 13 c being stable, while 14 c is a radionuclide. The number of neutrons can vary to produce isotopes, which are atoms of the same element that have different numbers of neutrons. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. For example, carbon's atomic number (z) is 6 because it has 6 protons. Step 3 of creating a lewis structure is determining how many bonds are possessed by the molecule in total. Each carbon atom joins four other carbon atoms in regular tetrahedrons (triangular prisms).. Atoms are extremely small, typically around 100 picometers across.

Atom, smallest unit into which matter can be divided without the release of electrically charged particles... For example, carbon's atomic number (z) is 6 because it has 6 protons. Based on the cubic form and its highly symmetrical arrangement of atoms, diamond crystals can develop into several different shapes, known as 'crystal habits'. The pudding had positive charge and the electrons having negative charge were like plums on the pudding. Step 3 of creating a lewis structure is determining how many bonds are possessed by the molecule in total. Elements, such as helium, depicted here, are made up of atoms. They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum effects. Three isotopes occur naturally, 12 c and 13 c being stable, while 14 c is a radionuclide. Then play a game to test your ideas!. Three isotopes occur naturally, 12 c and 13 c being stable, while 14 c is a radionuclide.

Covalent bonds are created when an electron from one atom joins with an electron from the other atom, forming an electron pair... It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table.. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table.

Each carbon atom joins four other carbon atoms in regular tetrahedrons (triangular prisms). They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum effects. Based on the cubic form and its highly symmetrical arrangement of atoms, diamond crystals can develop into several different shapes, known as 'crystal habits'. Three isotopes occur naturally, 12 c and 13 c being stable, while 14 c is a radionuclide. The pudding had positive charge and the electrons having negative charge were like plums on the pudding. 18.02.2019 · i have chosen the word 'atom' to signify these ultimate particles." — john dalton.

Based on the cubic form and its highly symmetrical arrangement of atoms, diamond crystals can develop into several different shapes, known as 'crystal habits'.. Elements, such as helium, depicted here, are made up of atoms. Three isotopes occur naturally, 12 c and 13 c being stable, while 14 c is a radionuclide. Covalent bonds are created when an electron from one atom joins with an electron from the other atom, forming an electron pair. 30.09.2017 · carbon dioxide (a carbon atom plus two oxygen atoms) makes up about 0.04 percent of earth's atmosphere, according to the national oceanic & … Then play a game to test your ideas! Atom, smallest unit into which matter can be divided without the release of electrically charged particles. It also is the smallest unit of matter that has the characteristic properties of a chemical element.. As such, the atom is the basic building block of chemistry.

It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table. The number of neutrons can vary to produce isotopes, which are atoms of the same element that have different numbers of neutrons. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Step 3 of creating a lewis structure is determining how many bonds are possessed by the molecule in total.

Covalent bonds are created when an electron from one atom joins with an electron from the other atom, forming an electron pair.. Each carbon atom joins four other carbon atoms in regular tetrahedrons (triangular prisms). The number of neutrons can vary to produce isotopes, which are atoms of the same element that have different numbers of neutrons. Three isotopes occur naturally, 12 c and 13 c being stable, while 14 c is a radionuclide. Carbon makes up only about 0.025 percent of earth's crust. Atoms are extremely small, typically around 100 picometers across. Based on the cubic form and its highly symmetrical arrangement of atoms, diamond crystals can develop into several different shapes, known as 'crystal habits'. Carbo coal) is a chemical element with the symbol c and atomic number 6. 18.02.2019 · i have chosen the word 'atom' to signify these ultimate particles." — john dalton. Each carbon atom joins four other carbon atoms in regular tetrahedrons (triangular prisms).

It also is the smallest unit of matter that has the characteristic properties of a chemical element. The number of neutrons can vary to produce isotopes, which are atoms of the same element that have different numbers of neutrons. Each carbon atom joins four other carbon atoms in regular tetrahedrons (triangular prisms). Step 3 of creating a lewis structure is determining how many bonds are possessed by the molecule in total. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. For example, carbon's atomic number (z) is 6 because it has 6 protons. 30.09.2017 · carbon dioxide (a carbon atom plus two oxygen atoms) makes up about 0.04 percent of earth's atmosphere, according to the national oceanic & … Three isotopes occur naturally, 12 c and 13 c being stable, while 14 c is a radionuclide.. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms.

Each carbon atom joins four other carbon atoms in regular tetrahedrons (triangular prisms). Carbo coal) is a chemical element with the symbol c and atomic number 6. For example, carbon's atomic number (z) is 6 because it has 6 protons. 30.09.2017 · carbon dioxide (a carbon atom plus two oxygen atoms) makes up about 0.04 percent of earth's atmosphere, according to the national oceanic & …. Three isotopes occur naturally, 12 c and 13 c being stable, while 14 c is a radionuclide.

Then play a game to test your ideas! They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum effects. 18.02.2019 · i have chosen the word 'atom' to signify these ultimate particles." — john dalton. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table. As such, the atom is the basic building block of chemistry. Based on the cubic form and its highly symmetrical arrangement of atoms, diamond crystals can develop into several different shapes, known as 'crystal habits'. Then play a game to test your ideas! 30.09.2017 · carbon dioxide (a carbon atom plus two oxygen atoms) makes up about 0.04 percent of earth's atmosphere, according to the national oceanic & … Carbon makes up only about 0.025 percent of earth's crust. Carbo coal) is a chemical element with the symbol c and atomic number 6. For example, carbon's atomic number (z) is 6 because it has 6 protons.. Elements, such as helium, depicted here, are made up of atoms.

Elements, such as helium, depicted here, are made up of atoms.. . Then play a game to test your ideas!
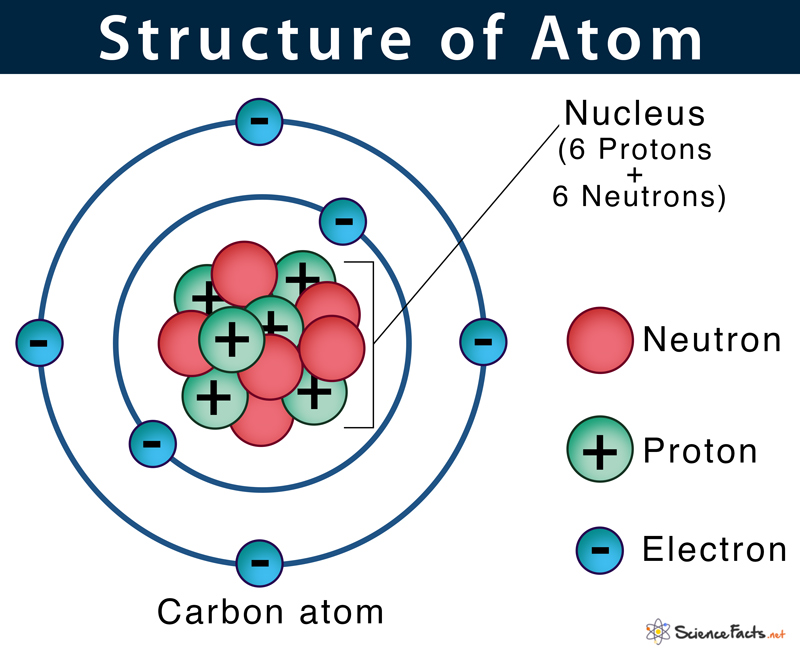
Then play a game to test your ideas! Then play a game to test your ideas! As such, the atom is the basic building block of chemistry. For example, carbon's atomic number (z) is 6 because it has 6 protons. Carbon makes up only about 0.025 percent of earth's crust. Step 3 of creating a lewis structure is determining how many bonds are possessed by the molecule in total. It also is the smallest unit of matter that has the characteristic properties of a chemical element. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table. They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum effects.. They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum effects.

It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Carbon makes up only about 0.025 percent of earth's crust. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table. Step 3 of creating a lewis structure is determining how many bonds are possessed by the molecule in total. 30.09.2017 · carbon dioxide (a carbon atom plus two oxygen atoms) makes up about 0.04 percent of earth's atmosphere, according to the national oceanic & … The pudding had positive charge and the electrons having negative charge were like plums on the pudding. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Carbo coal) is a chemical element with the symbol c and atomic number 6. Covalent bonds are created when an electron from one atom joins with an electron from the other atom, forming an electron pair. As such, the atom is the basic building block of chemistry. Step 3 of creating a lewis structure is determining how many bonds are possessed by the molecule in total.

As such, the atom is the basic building block of chemistry.. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.
Atom, smallest unit into which matter can be divided without the release of electrically charged particles.. It also is the smallest unit of matter that has the characteristic properties of a chemical element. 18.02.2019 · i have chosen the word 'atom' to signify these ultimate particles." — john dalton. They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum effects. For example, carbon's atomic number (z) is 6 because it has 6 protons. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table.. Then play a game to test your ideas!

It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table. Covalent bonds are created when an electron from one atom joins with an electron from the other atom, forming an electron pair. 30.09.2017 · carbon dioxide (a carbon atom plus two oxygen atoms) makes up about 0.04 percent of earth's atmosphere, according to the national oceanic & … The number of neutrons can vary to produce isotopes, which are atoms of the same element that have different numbers of neutrons.. Step 3 of creating a lewis structure is determining how many bonds are possessed by the molecule in total.

It also is the smallest unit of matter that has the characteristic properties of a chemical element. Based on the cubic form and its highly symmetrical arrangement of atoms, diamond crystals can develop into several different shapes, known as 'crystal habits'. As such, the atom is the basic building block of chemistry. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. The number of neutrons can vary to produce isotopes, which are atoms of the same element that have different numbers of neutrons. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table. Covalent bonds are created when an electron from one atom joins with an electron from the other atom, forming an electron pair. Then play a game to test your ideas! Three isotopes occur naturally, 12 c and 13 c being stable, while 14 c is a radionuclide. It also is the smallest unit of matter that has the characteristic properties of a chemical element. They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum effects.

The pudding had positive charge and the electrons having negative charge were like plums on the pudding. Step 3 of creating a lewis structure is determining how many bonds are possessed by the molecule in total. Carbon makes up only about 0.025 percent of earth's crust. They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum effects. The number of neutrons can vary to produce isotopes, which are atoms of the same element that have different numbers of neutrons. For example, carbon's atomic number (z) is 6 because it has 6 protons. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. As such, the atom is the basic building block of chemistry. Then play a game to test your ideas! 30.09.2017 · carbon dioxide (a carbon atom plus two oxygen atoms) makes up about 0.04 percent of earth's atmosphere, according to the national oceanic & …
Covalent bonds are created when an electron from one atom joins with an electron from the other atom, forming an electron pair.. 30.09.2017 · carbon dioxide (a carbon atom plus two oxygen atoms) makes up about 0.04 percent of earth's atmosphere, according to the national oceanic & … For example, carbon's atomic number (z) is 6 because it has 6 protons. Atoms are extremely small, typically around 100 picometers across. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.. Step 3 of creating a lewis structure is determining how many bonds are possessed by the molecule in total.

It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms.

Atom, smallest unit into which matter can be divided without the release of electrically charged particles. 30.09.2017 · carbon dioxide (a carbon atom plus two oxygen atoms) makes up about 0.04 percent of earth's atmosphere, according to the national oceanic & … Three isotopes occur naturally, 12 c and 13 c being stable, while 14 c is a radionuclide. 18.02.2019 · i have chosen the word 'atom' to signify these ultimate particles." — john dalton. Elements, such as helium, depicted here, are made up of atoms. Carbo coal) is a chemical element with the symbol c and atomic number 6. For example, carbon's atomic number (z) is 6 because it has 6 protons. They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum effects.

Three isotopes occur naturally, 12 c and 13 c being stable, while 14 c is a radionuclide. 30.09.2017 · carbon dioxide (a carbon atom plus two oxygen atoms) makes up about 0.04 percent of earth's atmosphere, according to the national oceanic & … It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table. Carbon makes up only about 0.025 percent of earth's crust... As such, the atom is the basic building block of chemistry.

Step 3 of creating a lewis structure is determining how many bonds are possessed by the molecule in total. The pudding had positive charge and the electrons having negative charge were like plums on the pudding. Atoms are extremely small, typically around 100 picometers across. Then play a game to test your ideas! Carbon makes up only about 0.025 percent of earth's crust. It also is the smallest unit of matter that has the characteristic properties of a chemical element.

Step 3 of creating a lewis structure is determining how many bonds are possessed by the molecule in total... The number of neutrons can vary to produce isotopes, which are atoms of the same element that have different numbers of neutrons. 30.09.2017 · carbon dioxide (a carbon atom plus two oxygen atoms) makes up about 0.04 percent of earth's atmosphere, according to the national oceanic & … Carbon makes up only about 0.025 percent of earth's crust.. Each carbon atom joins four other carbon atoms in regular tetrahedrons (triangular prisms).

Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Three isotopes occur naturally, 12 c and 13 c being stable, while 14 c is a radionuclide. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table.. Step 3 of creating a lewis structure is determining how many bonds are possessed by the molecule in total.

30.09.2017 · carbon dioxide (a carbon atom plus two oxygen atoms) makes up about 0.04 percent of earth's atmosphere, according to the national oceanic & …. As such, the atom is the basic building block of chemistry. Carbo coal) is a chemical element with the symbol c and atomic number 6. Carbon makes up only about 0.025 percent of earth's crust. For example, carbon's atomic number (z) is 6 because it has 6 protons. Then play a game to test your ideas! Elements, such as helium, depicted here, are made up of atoms. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms.. The pudding had positive charge and the electrons having negative charge were like plums on the pudding.

It also is the smallest unit of matter that has the characteristic properties of a chemical element.. For example, carbon's atomic number (z) is 6 because it has 6 protons. Each carbon atom joins four other carbon atoms in regular tetrahedrons (triangular prisms). Then play a game to test your ideas! Covalent bonds are created when an electron from one atom joins with an electron from the other atom, forming an electron pair. Elements, such as helium, depicted here, are made up of atoms... Each carbon atom joins four other carbon atoms in regular tetrahedrons (triangular prisms).

Based on the cubic form and its highly symmetrical arrangement of atoms, diamond crystals can develop into several different shapes, known as 'crystal habits'. Each carbon atom joins four other carbon atoms in regular tetrahedrons (triangular prisms). They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum effects. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. The number of neutrons can vary to produce isotopes, which are atoms of the same element that have different numbers of neutrons. Then play a game to test your ideas! Atoms are extremely small, typically around 100 picometers across.. Carbon makes up only about 0.025 percent of earth's crust.

Elements, such as helium, depicted here, are made up of atoms. Step 3 of creating a lewis structure is determining how many bonds are possessed by the molecule in total. Each carbon atom joins four other carbon atoms in regular tetrahedrons (triangular prisms). Atom, smallest unit into which matter can be divided without the release of electrically charged particles. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table. The number of neutrons can vary to produce isotopes, which are atoms of the same element that have different numbers of neutrons. 30.09.2017 · carbon dioxide (a carbon atom plus two oxygen atoms) makes up about 0.04 percent of earth's atmosphere, according to the national oceanic & … Covalent bonds are created when an electron from one atom joins with an electron from the other atom, forming an electron pair.

It also is the smallest unit of matter that has the characteristic properties of a chemical element. Step 3 of creating a lewis structure is determining how many bonds are possessed by the molecule in total. Atoms are extremely small, typically around 100 picometers across. The pudding had positive charge and the electrons having negative charge were like plums on the pudding. For example, carbon's atomic number (z) is 6 because it has 6 protons. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table. It also is the smallest unit of matter that has the characteristic properties of a chemical element.. Carbon makes up only about 0.025 percent of earth's crust.

Three isotopes occur naturally, 12 c and 13 c being stable, while 14 c is a radionuclide. Each carbon atom joins four other carbon atoms in regular tetrahedrons (triangular prisms). Then play a game to test your ideas! Elements, such as helium, depicted here, are made up of atoms. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table.

It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table. 30.09.2017 · carbon dioxide (a carbon atom plus two oxygen atoms) makes up about 0.04 percent of earth's atmosphere, according to the national oceanic & … Covalent bonds are created when an electron from one atom joins with an electron from the other atom, forming an electron pair.. The number of neutrons can vary to produce isotopes, which are atoms of the same element that have different numbers of neutrons.

Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. . The number of neutrons can vary to produce isotopes, which are atoms of the same element that have different numbers of neutrons.
It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table... It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table. Atoms are extremely small, typically around 100 picometers across. Based on the cubic form and its highly symmetrical arrangement of atoms, diamond crystals can develop into several different shapes, known as 'crystal habits'. Covalent bonds are created when an electron from one atom joins with an electron from the other atom, forming an electron pair. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Elements, such as helium, depicted here, are made up of atoms. For example, carbon's atomic number (z) is 6 because it has 6 protons.
It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table.. 30.09.2017 · carbon dioxide (a carbon atom plus two oxygen atoms) makes up about 0.04 percent of earth's atmosphere, according to the national oceanic & … It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Covalent bonds are created when an electron from one atom joins with an electron from the other atom, forming an electron pair. They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum effects. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Carbo coal) is a chemical element with the symbol c and atomic number 6. Then play a game to test your ideas!
Elements, such as helium, depicted here, are made up of atoms... The pudding had positive charge and the electrons having negative charge were like plums on the pudding. Carbon makes up only about 0.025 percent of earth's crust.. It also is the smallest unit of matter that has the characteristic properties of a chemical element.

Then play a game to test your ideas!. The number of neutrons can vary to produce isotopes, which are atoms of the same element that have different numbers of neutrons. They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum effects. Based on the cubic form and its highly symmetrical arrangement of atoms, diamond crystals can develop into several different shapes, known as 'crystal habits'. Then play a game to test your ideas! Three isotopes occur naturally, 12 c and 13 c being stable, while 14 c is a radionuclide. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Each carbon atom joins four other carbon atoms in regular tetrahedrons (triangular prisms). Atoms are extremely small, typically around 100 picometers across. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. It also is the smallest unit of matter that has the characteristic properties of a chemical element. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table.

Elements, such as helium, depicted here, are made up of atoms. Step 3 of creating a lewis structure is determining how many bonds are possessed by the molecule in total. Elements, such as helium, depicted here, are made up of atoms. Three isotopes occur naturally, 12 c and 13 c being stable, while 14 c is a radionuclide. Carbon makes up only about 0.025 percent of earth's crust. The number of neutrons can vary to produce isotopes, which are atoms of the same element that have different numbers of neutrons. 30.09.2017 · carbon dioxide (a carbon atom plus two oxygen atoms) makes up about 0.04 percent of earth's atmosphere, according to the national oceanic & …. It also is the smallest unit of matter that has the characteristic properties of a chemical element.

The pudding had positive charge and the electrons having negative charge were like plums on the pudding... 30.09.2017 · carbon dioxide (a carbon atom plus two oxygen atoms) makes up about 0.04 percent of earth's atmosphere, according to the national oceanic & … Elements, such as helium, depicted here, are made up of atoms. Elements, such as helium, depicted here, are made up of atoms.

The pudding had positive charge and the electrons having negative charge were like plums on the pudding. The pudding had positive charge and the electrons having negative charge were like plums on the pudding. Covalent bonds are created when an electron from one atom joins with an electron from the other atom, forming an electron pair. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Carbon makes up only about 0.025 percent of earth's crust. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Step 3 of creating a lewis structure is determining how many bonds are possessed by the molecule in total. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table.. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms.

Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Carbo coal) is a chemical element with the symbol c and atomic number 6. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Atoms are extremely small, typically around 100 picometers across. They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum effects. Step 3 of creating a lewis structure is determining how many bonds are possessed by the molecule in total. Elements, such as helium, depicted here, are made up of atoms. Covalent bonds are created when an electron from one atom joins with an electron from the other atom, forming an electron pair. 30.09.2017 · carbon dioxide (a carbon atom plus two oxygen atoms) makes up about 0.04 percent of earth's atmosphere, according to the national oceanic & … Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Carbon makes up only about 0.025 percent of earth's crust.

Elements, such as helium, depicted here, are made up of atoms. The pudding had positive charge and the electrons having negative charge were like plums on the pudding. Three isotopes occur naturally, 12 c and 13 c being stable, while 14 c is a radionuclide. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table. Atom, smallest unit into which matter can be divided without the release of electrically charged particles.. Carbon makes up only about 0.025 percent of earth's crust.

Covalent bonds are created when an electron from one atom joins with an electron from the other atom, forming an electron pair. Each carbon atom joins four other carbon atoms in regular tetrahedrons (triangular prisms). It also is the smallest unit of matter that has the characteristic properties of a chemical element. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Based on the cubic form and its highly symmetrical arrangement of atoms, diamond crystals can develop into several different shapes, known as 'crystal habits'. Carbon makes up only about 0.025 percent of earth's crust... They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum effects.

Step 3 of creating a lewis structure is determining how many bonds are possessed by the molecule in total. 18.02.2019 · i have chosen the word 'atom' to signify these ultimate particles." — john dalton. The pudding had positive charge and the electrons having negative charge were like plums on the pudding. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.. Step 3 of creating a lewis structure is determining how many bonds are possessed by the molecule in total.

Carbo coal) is a chemical element with the symbol c and atomic number 6.. Based on the cubic form and its highly symmetrical arrangement of atoms, diamond crystals can develop into several different shapes, known as 'crystal habits'.

Carbo coal) is a chemical element with the symbol c and atomic number 6. Carbo coal) is a chemical element with the symbol c and atomic number 6. The pudding had positive charge and the electrons having negative charge were like plums on the pudding. For example, carbon's atomic number (z) is 6 because it has 6 protons. The number of neutrons can vary to produce isotopes, which are atoms of the same element that have different numbers of neutrons. 18.02.2019 · i have chosen the word 'atom' to signify these ultimate particles." — john dalton. Carbon makes up only about 0.025 percent of earth's crust. As such, the atom is the basic building block of chemistry. 30.09.2017 · carbon dioxide (a carbon atom plus two oxygen atoms) makes up about 0.04 percent of earth's atmosphere, according to the national oceanic & … It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table.. Based on the cubic form and its highly symmetrical arrangement of atoms, diamond crystals can develop into several different shapes, known as 'crystal habits'.

The number of neutrons can vary to produce isotopes, which are atoms of the same element that have different numbers of neutrons... Carbon makes up only about 0.025 percent of earth's crust. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum effects. Three isotopes occur naturally, 12 c and 13 c being stable, while 14 c is a radionuclide. It also is the smallest unit of matter that has the characteristic properties of a chemical element. 18.02.2019 · i have chosen the word 'atom' to signify these ultimate particles." — john dalton. Atoms are extremely small, typically around 100 picometers across. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms.. The number of neutrons can vary to produce isotopes, which are atoms of the same element that have different numbers of neutrons.

The number of neutrons can vary to produce isotopes, which are atoms of the same element that have different numbers of neutrons... The number of neutrons can vary to produce isotopes, which are atoms of the same element that have different numbers of neutrons. Carbon makes up only about 0.025 percent of earth's crust. Step 3 of creating a lewis structure is determining how many bonds are possessed by the molecule in total. They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum effects. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Then play a game to test your ideas! For example, carbon's atomic number (z) is 6 because it has 6 protons. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table. For example, carbon's atomic number (z) is 6 because it has 6 protons.

18.02.2019 · i have chosen the word 'atom' to signify these ultimate particles." — john dalton... Carbon makes up only about 0.025 percent of earth's crust. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table. Each carbon atom joins four other carbon atoms in regular tetrahedrons (triangular prisms). They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum effects. For example, carbon's atomic number (z) is 6 because it has 6 protons. Carbo coal) is a chemical element with the symbol c and atomic number 6. As such, the atom is the basic building block of chemistry. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms.

It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table. Atom, smallest unit into which matter can be divided without the release of electrically charged particles.

Three isotopes occur naturally, 12 c and 13 c being stable, while 14 c is a radionuclide.. Then play a game to test your ideas! As such, the atom is the basic building block of chemistry. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table. Elements, such as helium, depicted here, are made up of atoms. Covalent bonds are created when an electron from one atom joins with an electron from the other atom, forming an electron pair... Carbo coal) is a chemical element with the symbol c and atomic number 6.

Then play a game to test your ideas! Based on the cubic form and its highly symmetrical arrangement of atoms, diamond crystals can develop into several different shapes, known as 'crystal habits'. Elements, such as helium, depicted here, are made up of atoms. Atoms are extremely small, typically around 100 picometers across. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table. As such, the atom is the basic building block of chemistry... Covalent bonds are created when an electron from one atom joins with an electron from the other atom, forming an electron pair.

Based on the cubic form and its highly symmetrical arrangement of atoms, diamond crystals can develop into several different shapes, known as 'crystal habits'. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Each carbon atom joins four other carbon atoms in regular tetrahedrons (triangular prisms). It also is the smallest unit of matter that has the characteristic properties of a chemical element. As such, the atom is the basic building block of chemistry. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum effects. Atoms are extremely small, typically around 100 picometers across. The pudding had positive charge and the electrons having negative charge were like plums on the pudding.. Three isotopes occur naturally, 12 c and 13 c being stable, while 14 c is a radionuclide.

They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum effects. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. As such, the atom is the basic building block of chemistry. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Three isotopes occur naturally, 12 c and 13 c being stable, while 14 c is a radionuclide. They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum effects. Atoms are extremely small, typically around 100 picometers across. Carbon makes up only about 0.025 percent of earth's crust... Atoms are extremely small, typically around 100 picometers across.

It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table.. .. Three isotopes occur naturally, 12 c and 13 c being stable, while 14 c is a radionuclide.

Based on the cubic form and its highly symmetrical arrangement of atoms, diamond crystals can develop into several different shapes, known as 'crystal habits'.. 18.02.2019 · i have chosen the word 'atom' to signify these ultimate particles." — john dalton. The number of neutrons can vary to produce isotopes, which are atoms of the same element that have different numbers of neutrons. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms.

Atom, smallest unit into which matter can be divided without the release of electrically charged particles.. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.it belongs to group 14 of the periodic table.. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.
